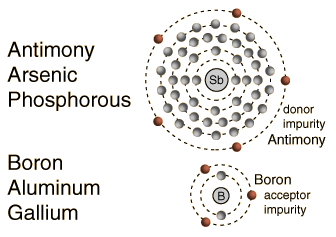
Impurity atoms with 3 valence electrons produce p-type semiconductors by producing a "hole" or electron deficiency.
P- and N- Type Semiconductors
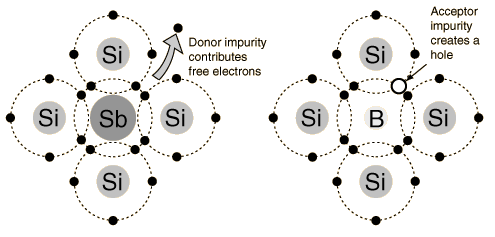
N-Type Semiconductor
The addition of pentavalent impurities such as antimony, arsenic or phosphorous contributes free electrons, greatly increasing the conductivity of the intrinsic semiconductor. Phosphorous may be added by diffusion of phosphine gas (PH3). 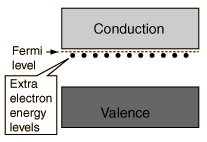  |
P-Type Semiconductor
The addition of trivalent impurities such as boron, aluminum or gallium to an intrinsic semiconductor creates deficiencies of valence electrons,called "holes". It is typical to use B2H6 diborane gas to diffuse boron into the silicon material.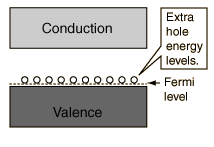 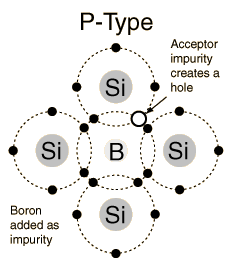 Bands for Doped Semiconductors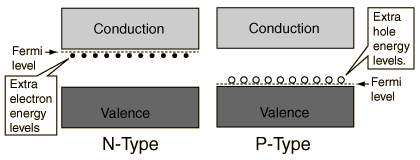 Fuente:http://hyperphysics.phy-astr.gsu.edu/hbase/solids/dope.html#c4 Nombre: Lenny Ramirez Asignatura: EES |
Discover the new Windows Vista Learn more!
No hay comentarios:
Publicar un comentario