A useful way to visualize the difference between conductors, insulators and semiconductors is to plot the available energies for electrons in the materials. Instead of having discrete energies as in the case of free atoms, the available energy states form bands. Crucial to the conduction process is whether or not there are electrons in the conduction band. In insulators the electrons in the valence band are separated by a large gap from the conduction band, in conductors like metals the valence band overlaps the conduction band, and in semiconductors there is a small enough gap between the valence and conduction bands that thermal or other excitations can bridge the gap. With such a small gap, the presence of a small percentage of a doping material can increase conductivity dramatically.
An important parameter in the band theory is the Fermi level, the top of the available electron energy levels at low temperatures. The position of the Fermi level with the relation to the conduction band is a crucial factor in determining electrical properties.
Energy Bands for Solids
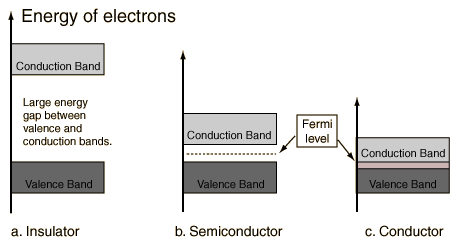
Energy Bands Comments
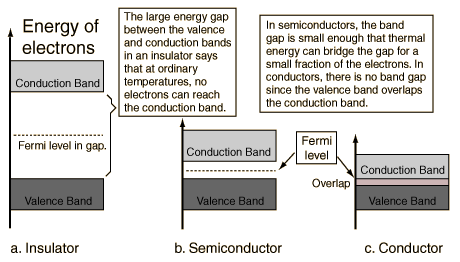
Semiconductor Energy Bands
| For intrinsic semiconductors like silicon and germanium, the Fermi level is essentially halfway between the valence and conduction bands. Although no conduction occurs at 0 K, at higher temperatures a finite number of electrons can reach the conduction band and provide some current. In doped semiconductors, extra energy levels are added. The increase in conductivity with temperature can be modeled in terms of the Fermi function, which allows one to calculate the population of the conduction band. 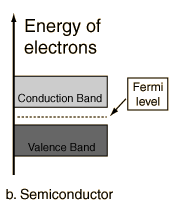 Fuente: http://hyperphysics.phy-astr.gsu.edu/hbase/solids/band.html#c1 Nombre: Lenny Ramirez Asignatura: EES |
Get news, entertainment and everything you care about at Live.com. Check it out!
No hay comentarios:
Publicar un comentario